Sulfur Supplying
Introduction
Sulfur
Brimstone
a bright yellow element
There is an important ingredient in oil and coal compounds (organosulfur), in natural gas (H2S(g)), and mineral sulfides and sulfates; which was classified as an sulfur element in 1777 by Lavoisier.
Following strontium, sulfur is the seventeenth most abundant element since it is 0.0384% of the Earth’s crust.
From the characteristics of pure sulfur, we can mention the tasteless and odorless with a light yellow color of this element
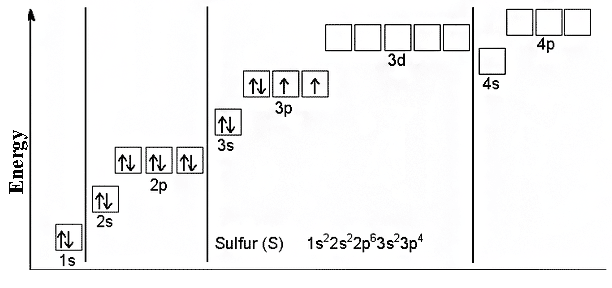
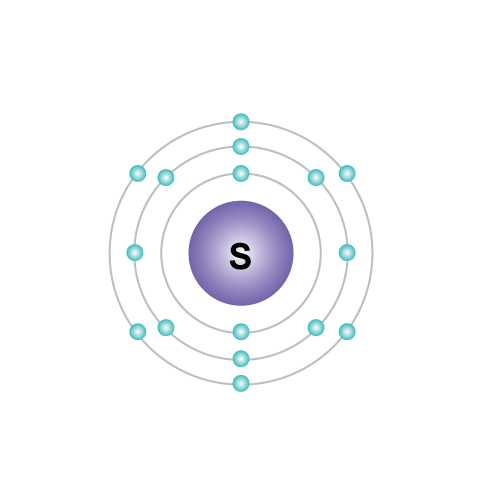
Physical and chemical properties
- Solid
- nonmetal
- 112 ˚C
- 444/6 ˚C
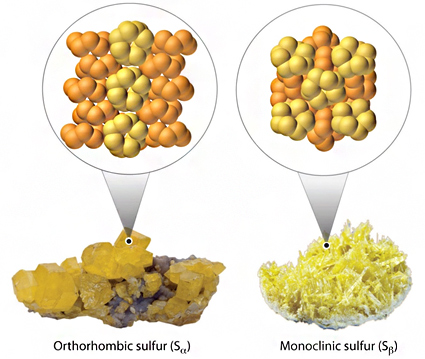
- Orthorhombic
- Amorphous
- 7704-34-9
- Phase at room temperature:
- Classification:
- Melting point:
- Boiling point:
- Crystal structure:
- Conductivity:
- CAS:
- Sulphur (British spelling)r
- S
- 32/07 gr/mol
- lemon yellow sintered microcrystals
- 16
- Chalcogens(16)
- 3
- P-block
- Alternative name:
- Atomic Symbol:
- Atomic Weight:
- Appearance:
- Atomic number(Z):
- group:
- period:
- Block:
:How to produce
Most sulfur obtained through superheated water injection in underground deposits and piping out molten sulfur directly ( Frasch process ).
During this method sulfur be made with high purity up to 99.9 percent.
The second and third source for sulfur extraction can be oil and natural gas through H2S reduction reactions.
In addition to the fact that sulfur has some isotopes, also many of its allotropes have also been found in the nature.
Sulfur and its reaction
Sulfur Oxides
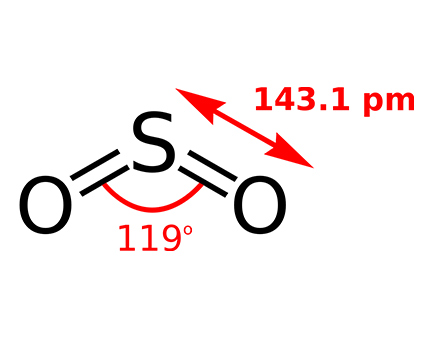
Among the different stable sulfur oxides, Sulfur dioxide and sulfur trioxide are commonly found.
Sulfur dioxide is a colorless gas which is pungent, and nonflammable (d= 2.8 kg/m3 m.p= -72.5 ˚C )
The liquid form of SO2 is used as a organic solvent that can solve organic compounds better than water.Also SO2 can be use as a precursor in SO3 production.
As you know the direct combustion of sulfur and the roasting of metal sulfides are the two main methods for the synthesis of SO2:
S(s)+O2(g)→SO2(g) Direct combustion
2ZnS(s)+3O2(g)→2ZnO(s)+2SO2 (g) Roasting of metal sulfides
The another sulfur derivative is sulfur trioxide soluble which like the SO2 is colorless ( d= 2.00 g/cm3 m.p= 62.3°C(α-), 32.5 °C(β-), 16.8 °C ( γ-form ) )
As mentioned before, SO3 obtain of SO2 and can be use in SO4 synthesis as a precursor.
2SO2(g)+O2(g)⇌2SO3(g) Exothermic, reversible reaction
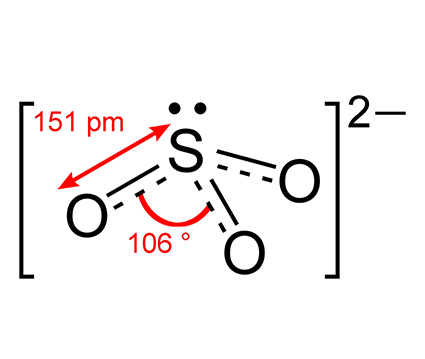
Hydrogen Sulfide H2S
H2S, Other names Dihydrogen monosulfide, Sour gas or Sulfane can define as a diprotic acid.
Equilibrium reactions of this acid:
H2S⇌HS−+H+
HS−⇌S2−+H+
Other Sulfur containing Compounds
Well known strong acid is sulfuric acid ( H2SO4 ) which is the most significant compound of sulfur used in modern industrialized societies as well.
SO3(g) + H2O(l) → H2SO4 (aq)
Safety and important points
- As possible it must keep away from water or even fog and keep container containing sulfur tightly closed in a cool, well-ventilated place.
- Sulfur is stable under normal conditions generally.
- When handling and transferring sulfur and its derivatives, in addition of wearing protective gloves/protective clothing/eye protection/face protection you have to avoid breathing dust, fume, gas, mist and vapors is necessary.
Applications
In chemicals production
Sulfuric acid, synthetic fibers, pigments, matches, explosives( including gunpowder), hydrogen sulfide, sulfur trioxide, thionyl chloride, carbon disulfide (an important organic solvent).
Application of sulfur in agriculture
Sulfur is an important component of plant protein and plays an important role in making metabolic compounds such as nitrogen, carbohydrate, and protein. It also plays a role in plant photosynthesis
Sulfur fertilizer, Pesticide, Fungicide and Reduces soil pH
In road construction
One of the material in Asphalt, asphalt concrete, calcium sulfate (gypsum) and cement production
In road construction
One of the material in Asphalt, asphalt concrete, calcium sulfate (gypsum) and cement production
In medicine
In the production of medicinal products, treatment of some skin diseases, Epsom salts () and exfoliants
In the food industry
Sulfite in the production of sugar, preservative compounds
In fabric production
Whitening of fabrics
In pulp and paper
Sodium sulfite for paper production, paper bleaching
Mercaptans
In detergents
In the preparation of surfactants and detergents, bleach and disinfectant
For more information about sulfur and its properties we offer ” SULFUR. HISTORY, TECHNOLOGY, APPLICATIONS AND INDUSTRY” 3RD Edition, frome Gerald Kutney, Published by ChemTec Publishing
we like to offer our customers